A Review on Anthraquinones Isolated From Cassia Spcies Adn Their Applications

Potential Anti-Acetylcholinesterase Activity of Cassia timorensis DC.
1
School of Pharmaceutical Sciences, Universiti Sains Malaysia, 11800 Minden, Malaysia
2
USM-RIKEN Centre for Aging Science (URICAS), Universiti Sains Malaysia, 11800 Minden, Malaysia
3
Chemical Biology Research Grouping, RIKEN Centre for Sustainable Resources Science, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
*
Authors to whom correspondence should be addressed.
†
These authors contributed every bit to this piece of work.
Received: 1 September 2020 / Revised: 22 September 2020 / Accepted: 30 September 2020 / Published: 4 October 2020
Abstract
Seventeen methanol extracts from different plant parts of v different Cassia species, including C. timorensis, C. grandis, C. fistula, C. spectabilis, and C. alata were screened against acetylcholinesterase (Ache). C. timorensis extracts were plant to exhibit the highest inhibition towards Ache whereby the leaf, stem, and flower methanol extracts showed 94–97% inhibition. As far as we are aware, C. timorensis is one of the least explored Cassia spp. for bioactivity. Further fractionation led to the identification of six compounds, isolated for the first time from C. timorensis: 3-methoxyquercetin (1), benzenepropanoic acid (2), nine,12,15-octadecatrienoic acid (3), β-sitosterol (4), stigmasterol (5), and 1-octadecanol (6). Compound 1 showed moderate inhibition towards AChE (ICl: 83.71 μM), while the other compounds exhibited poor to slightly moderate Ache inhibitory activity. Molecular docking revealed that the methoxy substitution of 1 formed a hydrogen bond with TYR121 at the peripheral anionic site (PAS) and the hydroxyl group at C5 formed a covalent hydrogen bond with ASP72. Additionally, the OH group at the C3′ position formed an interaction with the protein at the acyl pocket (PHE288). This peradventure explains the activity of ane in blocking the entry of acetylcholine (ACh, the neurotransmitter), thus impeding the hydrolysis of ACh.
one. Introduction
Cassia is a huge genus of flowering plants belongs to the Fabaceae family which comprised of more than 500 species that are diverse in herbs, shrubs, and trees [i]. This genus is widely distributed in tropical countries such as the South-East asia, tropical America and African regions. Taxonomically, species of the Cassia genus are hands identified due to their brilliant yellowish, pinkish, and white flowers, along with the presence of legume fruits. Many species of Cassia accept medicinal values and they might differ from one to another depending on their phytochemical constituents [2]. For almost known Cassia species, the leaves, stems, pods, and seeds have been shown to possess dissimilar pharmacological values [iii,4]. Amongst the known ethnopharmacological applications of Cassia spp. are the treatment of pare diseases such as eczema, ringworm, scabies, and leprosy [5,vi,7]. Reported in vitro studies also showed their possible utilise as a handling for human brain disorders, such as epilepsy, migraine, and hysteria [eight,9].
Alzheimer's disease (AD) has go a major concern worldwide as no cure has been constitute still. To date, three clinical drugs have been canonical by the The states Food and Drug Administration (FDA), which are all cholinesterase inhibitors; donepezil, galanthamine, and rivastigmine [10]. AD is classified as a neurodegenerative disorder. It is characterized by consistent loss of neurons in the cognitive system, the appearance of amyloid plaques, intercellular hyper-phosphorylated neurofibrillary tangles (NFTs), and an inadequate level of acetylcholine (ACh), a neurotransmitter in the brain [11]. The acetylcholinesterase enzyme (Anguish) has an important function in the treatment of AD, where impediment of its activity (hydrolyzing the acetylcholine (ACh) neurotransmitter into choline and acetate) volition assistance to maintain the longevity of the neurotransmitter in the cognitive cortex [12,thirteen]. ACh transports signals within neurons and it significantly contributes to memory and learning. Over-catalysis of ACh by AChE will decrease the level of ACh in the brain specifically in the nucleus basalis of Meynert and eventually will worsen the AD symptoms [14]. At present, several natural compounds accept been tested for their power to inhibit Ache and among them are terpenes, coumarins, xanthones, isoquinolines, piperidine alkaloids [11,fifteen], anthraquinones [16] and flavonoids [17]. Thus far, Cassia species have been reported to exist abundant in anthraquinones and flavonoids, which are responsible for their ethno-pharmaceutical values [18,19,xx]. Of the known plants in the genus Cassia, Cassia timorensis is amidst the to the lowest degree explored. The pharmacological values of this item species are yet to be uncovered as evidenced by the lack of information in the literature. Cassia timorensis (DC.) H. Due south. Irwin & Barneby is currently considered as a synonym of Senna timoriensis (DC.) H. Due south. Irwin & Barneby as recorded in "The Institute List" website [21]. To date, only i compound has been reported in the literature that was isolated from C. timorensis. This compound known as barakol was isolated from the aqueous acerb acid extract of the leaves [22]. Barakol was also previously isolated from Cassia siamea and reported to have allaying and anxiolytic effects [23,24]. Here, we present the results of Ache inhibition of selected Cassia spp., which led united states of america towards the phytochemical investigation of C. timorensis. The isolated compounds were also tested for their anti-Ache action through in vitro and in silico studies in our quest to further understand the inhibition mechanism at the molecular level.
two. Results and Discussion
2.1. Inhibitory Action of Acetylcholinesterase by Cassia spp.
The inhibitory action of the methanol extracts of five Cassia spp., i.e., C. timorensis, C. grandis, C. fistula, C. spectabilis and C. alata, was carried out at 0.2 mg/mL and classed according to poor, moderate or adept inhibitions (poor: v–25%; moderate: 25–fifty%; good: l–100%); the results are presented in Table 1. From the 17 extracts, 6 showed positive results with the per centum of inhibition greater than 50% (adept inhibition). C. timorensis methanol extracts showed good inhibitory activeness, exhibiting the highest inhibitory percentage for extracts from leaves, stems, and flowers at 94.69%, 97.sixteen%, and 96.94%, respectively. C. grandis leaf and stem extracts similarly displayed proficient AChE inhibitory activeness with 86.09% and 91.76%, respectively. Apart from these two plants, simply the stems crude extract of C. fistula showed a practiced inhibitory action at 88.66%. Methanolic extracts that showed inhibition at <l%, or no inhibition towards AChE, were considered as moderate and poor inhibitors. Following these observations, we selected C. timorensis for further report.
The aqueous, butanol, and ethyl acetate fractions of C. timorensis leaf excerpt (Table 2) exhibited practiced inhibitory activeness with 98.80%, 91.83%, and 98.98% inhibition at 0.2 mg/mL, respectively. Likewise, hexane, ethyl acetate, and butanol fractions from C. timorensis flowers as well exhibited expert inhibitory activity with 92.35%, 95.98%, and 98.16%, respectively. On the other hand, for the stems, the ethyl acetate fraction just showed xxx.84% inhibitory activity, while the butanol and aqueous fractions showed good activity with 94.92 and 84.35% inhibition, respectively. We postulated that ethyl acetate might yield semi-polar compounds, which might exist able to cross the claret-brain barrier better than the polar compounds every bit suggested by Li and co-workers [25]. Therefore, nosotros selected the ethyl acetate fractions of the leaves and flowers of C. timorensis for ICfifty determination. The stalk was not selected every bit its ethyl acetate did non testify encouraging inhibitory activeness against AChE. The ICfifty of both the leaves and flowers' ethyl acetate fractions seemed promising (IC50 of 19.21 ± iv.sixteen µg/mL (leaves) and 12.75 ± 6.28 µg/mL (flowers)). This encouraged the states to farther attempt to isolate compounds from the ethyl acetate fractions of this Cassia spp. In addition, the hexane fraction of the flowers also demonstrated good inhibition towards Anguish; thus, we also selected the hexane fractions of these establish parts in our isolation report (IC50 of the hexane fraction of the bloom is 12.98 ± 0.95 µg/mL).
2.2. Identification of Isolated Compounds
Yellowish needles of chemical compound 1 were isolated from the ethyl acetate fraction of C. timorensis leaves and characterized every bit iii-methoxyquercetin. Compound 1 or 3-methoxyquercetin, is a flavonol with a TLC retention gene (Rf) value of 0.62 (Hex/EtAc: 2:8). The 1H- and 13C-NMR spectra showed characteristic signals for flavonol skeletons with a methoxy grouping substitution at the C3 position. Compounds 2 and iii appeared as xanthous oil and were isolated from the hexane fraction of C. timorensis leaf extract. Compound 2, characterized as benzenepropanoic acid, was recently isolated from Trigonella foenum (Fabaceae) and demonstrated to accept anti-cancer potential through in vitro and in vivo studies [26]. To the best of our noesis, there was no report on the isolation of this compound from the genus Cassia, although it has been reported to be nowadays in C. fistula seeds as identified from GC-MS [27]. Compound 3 was characterized as 9,12,xv-octadecatrienoic acid, also known equally linolenic acid. Linolenic acid is a fat acid that can be found abundantly in well-nigh of the plants in the genus Cassia [28].
On the other mitt, white crystalline needles, with Rf values of 0.68 (EtAc/Hex: 3:vii) were isolated. On the footing of spectral data analysis equally well every bit past comparing the spectral data to literature data, compounds 4 and 5 have been established equally a mixture of β-sitosterol and stigmasterol. β-sitosterol and stigmasterol are common sterols in plants that often be in a mixture [29,30]. The but difference between these 2 compounds is that stigmasterol has an extra double bond at C-22 (δC 138.46) and C-23 (δC 129.42) over β-sitosterol. Studies have also shown that obtaining β-sitosterol or stigmasterol as a pure course is difficult [31]. Compound 6 isolated from the hexane fraction was characterized as one-octadecanol based on the analysis of NMR (1D/2d) and GC-MS data. Information technology has been previously isolated from Cassia sophera heartwood excerpt [32] and identified by gas chromatography (GC) techniques from various plants [33,34,35].
To the all-time of our cognition, this is the first report on the isolation of compounds 1–6 (Figure 1) from C. timorensis. The construction elucidation of the isolated compounds was done based on the UV, NMR (1D and 2d), and MS data too as by comparison to the published data (Supplementary data). The key correlations of COSY and HMBC of compounds 1–6 can be found in Effigy 2.
2.iii. In Vitro Acetylcholinesterase Enzyme Inhibition Action
The in vitro AChE inhibitory activity of the isolated compounds was evaluated at 0.1 mg/mL and their IC50 values were as well determined (Tabular array three). Compound 1 demonstrated AChE inhibition with an IC50 of 83.71 µM. However, the action of both ane and quercetin are quite depression compared to the standard drug galantamine (4.63 ± 0.03 µM). Compounds ii, three, and 6 were institute to have weak inhibitory activeness against AChE, while 4 and 5 demonstrated moderate activity against Anguish with 49.1% inhibition. Previous in vitro studies reported almost identical AChE inhibitory activities for compounds 4 and five, individually, where the IC50 of compound four was recorded at 55 µg/mL, while the ICfifty for compound v was 63 µg/mL [36]. Despite their individual notable inhibiting activities against Ache reported in the literature, we failed to separate them. The IC50 values of compounds 2–half dozen could non be determined due to their depression percent inhibition activity, which was less than 50% inhibition at 0.1 mg/mL.
Anguish inhibition of ane was adamant and compared with quercetin (of the same grade of compounds). The activity of 3-methoxyquercetin is approximately iii times higher than quercetin. The major departure between these 2 compounds based on their structure is only the substitution of the hydroxyl group at the C-3 position in quercetin with a methoxy grouping (–OCH3) in 1. A major debate involving neurological disorders is the ability of the drug candidates to penetrate the blood-brain barrier (BBB). Faria and colleagues conducted an in vivo written report on a rat endothelial RBE-iv cells BBB model and found that quercetin had the least efficiency in penetrating the BBB in a time-dependent manner compared to catechin and anthrocyanin due to its lower lipophilicity [37]. Permeability of a compound into the BBB is dependent on its lipophilicity. The less polar compounds, for example, the methylated flavonoids, are more than lipophilic and accept higher BBB permeability every bit compared to the more polar flavonoids. A contempo written report showed that sudachitin, aceronin, and nevadensin take high BBB permeability due to the exchange of a methyl group at both ring-A and ring-C on the two-phenyl chroman structure, which increases the lipophilicity of the compounds [38]. In some other study, the presence of a hydroxyl grouping (–OH) at the C-3 position did not give any impact on the inhibition of AChE [39]. In the present study, the methoxy group exchange at the C-3 position in ane did increment the inhibition of Ache as compared to the more than polar quercetin.
The evaluation of flavonoids as acetylcholinesterase inhibitors (AChEIs) has been widely investigated. In 2011, Uriarte-Pueyo and Calvo reported the ability of 128 flavonoid compounds, including those of subgroup classes such equally flavones, flavanones, flavonols, isoflavones, and chalcones as AChEIs. They found that the inhibitory activity of flavones is higher than the flavanones that have a double bond at C-2–C-3 of the C-ring position, which is important in impeding AChE action [forty]. The action of flavonoids as Ache inhibitors were also reported by Balkis and co-workers [41]. They concluded that the activity of the compounds depends on 4 main aspects: (1) the presence of an unsaturated two-phenyl chroman structure; (2) the presence of a hydroxyl group attached at C-5, C-six, and C-7 positions on the A-ring; (3) the insignificance of the hydroxyl group present in the B-ring, and (4) the presence of a substituted group at the C-3 position of the unsaturated C-ring [41]. 1 showed the feature of an unsaturated 2-phenyl chroman structure with the attachment of a hydroxyl group at C-5 and C-7. Importantly, ane has a exchange of a methoxy group at the C-3 position, which peradventure contributes to the inhibitory activity confronting AChE.
2.iv. In Silico Molecular Docking
The active site of the protein Anguish is characterized by a deep and narrow gorge, approximately twenty Å, that penetrates half of the Anguish and widens out to the base [42]. The entry gate of the active site is known as the peripheral anionic site (PAS) and consists of several important amino acids including TYR70, ASP72, TYR121, and TRP279 [43]. In addition, AChE is made upward of ii subsites; anionic and esteratic subsites that correspond to the choline-binding and catalytic pockets, respectively. The anionic subsite consists of an important amino acid, TRP84, which is highly conserved. TRP84 is i of the near significant amino acids in the Anguish binding pocket for the hydrophobic cationic-π interaction to occur. Meanwhile, the esteratic subsite has 2 binding pockets; the oxyanion hole (GLY116, GLY117 and ALA199) and the acyl pocket (PHE330 and PHE 331), which are responsible for the stabilization of the substrate and then that hydrolyzation can take place. The esteractic subsite is as well responsible for the hydrolysis of the ACh neurotransmitter at its catalytic triad (SER200-HIS440-GLU327) [44].
Docking assay of galantamine, one, and quercetin are presented in Figure 3. Chemical compound 1 jump to the AChE pocket with the lowest free energy of binding (FEB) of −8.43 kcal/mol, similarly to galantamine (−nine.63 kcal/mol). The 3D visualization analysis showed that i formed an interaction with the PAS, which includes several of import amino acids; ASP72, TYR121 and PHE288. TYR121 formed a hydrogen bond with the methoxy group located at the C-3 position of the C-ring with a altitude of 2.46 Å. A hydrogen bond was also formed between the hydrogen cantlet of the 7-OH group of the A-ring with the ASP72 at a altitude of 1.75 Å. Another hydrogen bail was formed between the oxygen atom 3′-OH of the B-ring with the acyl pocket (PHE288) of the agile site gorge with a distance of 2.78 Å. These three hydrogen bonds might explain the inhibitory activity of compound 1 as observed from the in vitro assay. In contrast, quercetin (−eight.38 kcal/mol) was institute to form two hydrogen bonds between 7-OH and TYR121 (PAS), and also hydrophobic interaction with ASP72 (PAS). Other interactions were observed from 3′-OH of the B-ring with TYR130 and a hydrophobic interaction with TRP84 at the choline binding site.
For comparison, galantamine bound to the active site pockets with the everyman Feb at −9.63 kcal/mol. A strong hydrogen bond between the hydroxyl oxygen of galantamine to the GLU199 was observed with a distance of 1.77 Å. Apart from that, the oxygen group from the methoxy group of galantamine formed a hydrogen bond with the anionic subsite amino acid (TYR130). The double bond of cyclohexene (C1=C2) of the galantamine faced toward the indole ring of TRP84 and stacked against the pi system of the TRP84, forming a favorable hydrophobic interaction. In improver, the oxygen atom from the methyl group and the oxygen atom of the tetrahydrofuran grouping of galantamine are involved in hydrogen bonding with both the catalytic residues (SER200 and HIS440). In addition, galantamine also aligned in a planar position and was stabilized via a hydrophobic interaction with the oxyanion hole and acyl pocket residues (GLY118, PHE330, and PHE331).
Based on the docking results, one was bound to PAS and sandwiched betwixt the acyl pocket of PHE288 halfway along the active site gorge. Based on a contempo finding by Neto and his colleagues, binding that occurs at PAS and the acyl pocket would obstruct the hydrolysis of neurotransmitters by blocking their pathway to the catalytic triad site located at the bottom of the active site [45]. Thus, the binding of 1 at this position will likely help to block the ACh neurotransmitters from entering the agile site, hence prolonging their activity and ultimately improving the symptoms of Advertising [44]. In contrast, the interactions of quercetin were found mainly between the B-ring of quercetin and the choline binding site (TYR130 and TRP84). All the same, as discussed before, any interaction that occurs between the hydroxyl group of the B-ring with Anguish active sites is not able to fully inhibit AChE [41].
On the other manus, galantamine primarily targets the anionic subsite and the esteratic subsite; (i) oxyanion pigsty, (two) acyl pocket, (3) catalytic triad. As observed, the flavonoid compounds, 1 and quercetin exert an inhibitory mechanism mainly at the PAS, acyl pocket, and choline binding site without any interaction with residues that brand upward the oxyanion pigsty and the catalytic triad. These docking results of both compounds and galantamine perhaps clarify the differences in the inhibitory action exhibited past these 3 compounds in our in vitro results.
three. Materials and Methods
three.1. Materials (Chemicals) and Instruments
Acetylcholinesterase (Anguish) from Electrophorus electricus (electric eels) type 6-South (510 units/mg), substrate acetylthiocholine iodide (ATCI), phosphate buffer, sodium phosphate monobasic and sodium phosphate dibasic, quercetin, and galantamine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Coloring agent five-5′dithiobis [ii-nitrobenzoicacid] (DTNB) was purchased from ACROS (Geel, Belgium). Silica gel (Kieselgel 60, 230–400 mesh atm) was purchased from Merck (Darmstadt, Germany). The solvents were of analytical grade and used as received.
NMR data were collected using either a Bruker Biospin spectrometer at 500 MHz for 1H and 125 MHz for thirteenC or Bruker advance III Hard disk 700 MHz NMR spectrometer, equipped with a 5-mm BBO probe, operating at 700 MHz for 1H and 175 MHz for 13C. Residual solvent signals were applied for referencing. MS data were acquired using a UPLC-H-Class organization (Waters, Millford, MA, Us) on a C18 cavalcade (i.d 2.ane mm × fifty mm, ane.7 µm particle sizes). The elution was done using a linear gradient organization (acetonitrile; 0.05% aqueous formic acid: 5 to 100% for 4 min at 0.five mL/min). The injected analytes absorbed UV and were detected by a UV/Vis detector, proportional to the pinnacle signals produced on the chromatogram. The analytes were then converted and fragmented into charged ions past the ESI interface and were brought into the mass spectrometer (Triple Quad™ LC-MS/MS system). A positive ion mode mass spectrum was generated. MS data were also obtained using GC (HP 6890 series GC system, Hewlett-Packard, Palo Alto, CA, USA), equipped with an autosampler (HP 7683 series injector) and coupled with a mass selective detector (HP 5973) using a cross-linked 5% phenylmethylsiloxane capillary column (thirty grand × 0.25 mm i.d., 0.25 moving-picture show thickness).
iii.2. Found Materials
Leaves, stems, fruits, and flowers of C. alata Fifty. Roxb were nerveless from Pahang, while C. timorensis (DC.) H. S Irwin & Barneby, C. spectabilis (DC.) H. S Irwin & Barneby, C. grandis L. f., and C. fistula L. were collected from Universiti Sains Malaysia (USM), and identified by a USM botanist, Mr. Shunmugan Vellosamy, from the Herbarium Department of the School of Biological Sciences, USM. The leaves, stems, and flowers were air-stale for seven days, while the fruits were freeze-stale for 72 h. Dried samples were powdered to increment surface surface area during the extraction procedure.
three.3. Extraction of Cassia Species for Screening
Two hundred grams of each institute sample was soaked in 99.ix% methanol for 3 days and filtered using Whatman filter paper no 1. The extraction process was performed iii times and the filtrates were pooled together. Then methanol was evaporated nether force per unit area at xl °C to obtain a rough methanolic extract. The crude extracts were stored at v °C prior to utilise.
iii.four. Collection, Extraction and Fractionation of C. timorensis
C. timorensis leaves and flowers collected from Universiti Sains Malaysia were identified and approved equally C. timorensis. The voucher specimen (No: 11778) was deposited in the Herbarium, School of Biological Sciences, Universiti Sains Malaysia. Briefly, air-stale leaves (1 kg) and flowers (300 one thousand) of C. timorensis were extracted by soaking in methanol at room temperature for three days followed past filtration using Whatman filter newspaper no. one to separate the found materials. This process was repeated thrice. So, the filtrates were combined and stale under reduced force per unit area at xl °C to yield methanolic extracts (100 1000 for leaves and 30 g for flowers). The crude extracts were stored at four °C until further use. The methanol extracts of C. timorensis (100 g for leaves and 30 g for flowers) were reconstituted in water:methanol (9:one) followed by liquid–liquid partitioning with n-hexane, ethyl acetate and butanol. Each fractionation was conducted iii times with 250 mL of the respected solvent. The fractions from the aforementioned solvent were brought together and dried nether vacuum before being screened for their anti-cholinesterase activity.
3.5. Isolation from the Ethyl Acetate Fraction of C. timorensis Leaves
The ethyl acetate fraction (14 grand) was purified by using column chromatography (silica gel 60, 230–400 mesh ASTM). The elution was washed with a stepwise gradient solvent system using chloroform:methanol, providing five major fractions; fraction 1 (100% chloroform), fraction ii (xc% chloroform), fraction 3 (85% chloroform), fraction 4 (80% chloroform) and fraction 5 (50% chloroform). Fraction 4 was further purified using column chromatography (silica gel threescore, 230–400 mesh ASTM) and eluted with a gradient solvent organisation of n-hexane:ethyl acetate (100% hexane up to 100% ethyl acetate v/v) to give five major sub-fractions. Purification of sub-fraction 2 yielded iii-methoxyquercetin (21.iv mg) (1).
three.6. Isolation from the n-Hexane Fraction of C. timorensis Leaves
The n-hexane fraction (0.844 g) was dissolved in hexane:acetone (i:1) and packed with silica gel into a Redisep® solid load cartridge. The sample was further separated using medium pressure liquid chromatography (MPLC) (CombiFlash Rf, Teledyne ISCO). The elution was performed with hexane (A): acetone (B) as the mobile phase in a stepwise gradient method (0–100% solvent B for 30 min) using a 24 m high performance gold silica column (RediSep® Rf, Teledyne ISCO). Five major fractions were collected. Fraction two and 4 were and so farther purified by using MPLC (xiii g C18 column, Redisep® Rf column) with a solvent system of acetonitrile:water (0.05% formic acid). Sub-fraction 1 from fraction two yielded benzenepropanoic acrid (2.2 mg), (two) whilst sub-fraction 7 from fraction 4 yielded octadecatrienoic acid (viii.iii mg) (3).
3.7. Isolation of Compounds of C. timorensis Flowers
The ethyl acetate fraction of C. timorensis flowers (6.00 yard) was subjected to a silica gel sixty (mesh 230–400 ASTM) cavalcade and eluted with a mixture of solvents of increasing polarity of n-hexane:ethyl acetate (100% hexane upwardly to 100% ethyl acetate (five/v)). The eluted fractions were monitored on TLC (silica gel sixty F254, Merck) using n-hexane:ethyl acetate. Fractions with similar TLC profiles were combined and pooled together to give thirty sub-fractions. Sub-fraction 9 was purified past crystallization, yielding white needle crystals. Based on spectral data assay also as by comparing to literature data, the crystals of sub-fraction 9 were identified as a mixture of β-sitosterol (12 mg) (4) and stigmasterol (12 mg) (5). Moreover, the n-hexane fraction of C. timorensis flowers (3.00 g) was subjected to column chromatography (silica gel 60, 230–400 ASTM) to yield 25 sub-fractions. The fractions were eluted using a slope mobile phase starting from 100% northward-hexane until 100% ethyl acetate was reached. Purification and crystallization of sub-fraction 8 gave chemical compound 6, which was identified as one-octadecanol (10 mg). On the other hand, purification and crystallization of sub-fraction 13 once again gave the mixture of sterol (compounds 4 and 5), which was isolated before from the ethyl acetate fraction.
3.eight. In Vitro Acetylcholinesterase Assay
The in vitro Ache inhibition assay was evaluated using a spectrophotometry method developed by Ellman with some modifications [41]. The assay was conducted in a 96-well plate with a total volume of 200 µL assay mixtures in comparison to galantamine every bit the positive command. Plant extracts were dissolved in dimethyl sulfoxide (DMSO) and tested at a final concentration of 0.2 mg/mL, while compounds were tested at 0.1 mg/mL. The final concentration of DMSO was fixed at 1% in the 96-well plate. In a 96-well plate, 178 µL of 50 mM phosphate buffer, two µL of 1 mg/mL compound and 20 mg/mL extract, and x µL of 0.5 U/mL Ache were added. The kickoff incubation period was 15 min at 25 °C. Five microliters of 14 mM ATCI substrate and 10 mM of the color indicator, DTNB, were added equally into each well and the well was and so incubated at 25 °C for thirty min to initiate the enzyme reaction. The absorbance was measured at 415 nm using Promega Glomax® Multi Plus Reader (Promega, Madison, WI, U.s.). Each sample was washed in triplicate and viii 2-fold serial dilutions were performed for each sample to determine the fifty-percentage inhibitory concentration (IC50). The percentage of inhibition was calculated using formula (i) [42]:
iii.ix. Statistical Assay
IC50 values of plant crude extract, fractions, and compounds were calculated from the graph of percentage of inhibition versus extract concentrations using Microsoft Excel. GraphPad Prism software was used to generate the graphs. The ICfifty values and the mean ± s.d. were calculated at 95% confidence interval.
3.10. In Silico Molecular Docking
The 3D structure of Torpedo californica acetylcholinesterase (TcAChE) in complex with galanthamine derivatives (PDB: 1W6R; 2.05 Å, [46]) was retrieved from Inquiry Collaboratory for Structural Bioinformatics (RCSB) Protein Data Banking company (http://www.rcsb.org/). The galantamine derivative leap in the crystal construction was taken out from the complex and saved as a PDB file using Discovery Studio 2.5 and assigned with Gasteiger charges using AutoDockTools 1.5.vi and subsequently redocked to the protein as a command docking using AutoDock4.2 [47]. Other ligands and water molecules present in the protein complex were also removed. Polar hydrogen atoms and Kollman charges were and then added to the protein. The grid box size was set to 90 × 90 × 90, with a filigree spacing of 0.375 Å, and the center of mass of the binding pocket was gear up to 3.518, 65.122, 64.481 (x, y, z, respectively). The docking parameter was set to default, assuasive 250 conformations by using the genetic algorithm search parameter. The 2D construction of galantamine, quercetin and one, were caused from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and converted to 3D using Discovery Studio 3.5, which was also used to visualize the 2D and 3D interactions for the lowest binding energy of the selected compounds with the bounden pocket of the TcAChE.
4. Conclusions
Screening of C. timorensis for acetylcholinesterase inhibition in this written report led to the identification of six compounds. As far as we are aware, all of these compounds were newly isolated from the plant since the first documented isolation of C. timorensis in 1984. The in vitro AChEI assay showed that compounds 2, 3, and 6 are not active, whilst 4 and v showed moderate activity as a mixture. Compound one was found to be active with an IC50 of 83.71 µM. The ability of 1 to inhibit Anguish in vitro was then simulated in silico against the targeted enzyme (TcAChE) to empathize the inhibitory mechanism at the molecular level. Chemical compound one displayed protein-ligand interactions at PAS and with the acyl pocket of the agile sites. The interaction at these positions volition likely obstruct the hydrolysis of the ACh neurotransmitter as it blocks the entrance and the go out from the enzyme agile sites.
Supplementary Materials
The following are available online. Scheme S1. Elution scheme for isolation of C.timorensis foliage and flowers extracts, Figure S1. Mass spectrum of 3-methoxy quercetin (1), Figure S2. iH-NMR of 3-methoxyquercetin (ane) [500 Hz, acetone-d6], Figure S3. thirteenC-NMR of 3-Methoxyquercetin (1) [125 MHz, acetone-d6], Figure S4. 1H→13C HSQC NMR spectrum of three-Methoxyquercetin (ane) [500 MHz, acetone-d6], Figure S5. 1H→13C HMBC NMR spectrum of 3-Methoxyquercetin (1) [500 MHz, acetone-d6], Figure S6. 1H→1H COSY NMR spectrum of 3-Methoxyquercetin (ane) [500 MHz, acetone-d6], Effigy S7. Mass spectrum of Benzenepropanoic acid (2), Figure S8. 1H-NMR of Benzenepropanoic acid (2) [500 MHz, CDCl3], Figure S9. 13C-NMR of Benzenepropanoic acrid (two) [125 MHz, CDCl3], Figure S10. 1H→13C HSQC NMR spectrum of Benzenepropanoic acid (2) [500 MHz, CDCl3], Figure S11. 1H→thirteenC HMBC NMR spectrum of Benzenepropanoic acid (2) [500 MHz, CDCl3], Figure S12. 1H→1H COSY NMR spectrum of Benzenepropanoic acid (two) [500 MHz, CDCl3], Figure S13. Mass spectrum of 9,12,15-Octadecatrienoic acid (three), Figure S14. 1H-NMR of 9,12,15-Octadecatrienoic acid (3) [500 MHz, CDCl3], Figure S15. xiiiC-NMR of 9,12,15-Octadecatrienoic acid (iii) [125 MHz, CDCl3], Figure S16. 1H→13C HSQC NMR spectrum of 9,12,15-Octadecatrienoic acrid (3) [500 MHz, CDCl3], Figure S17. 1H→13C HMBC NMR spectrum of 9,12,fifteen-Octadecatrienoic acrid (3) [500 MHz, CDCl3], Figure S18. 1H→1H COSY NMR spectrum of 9,12,15-Octadecatrienoic acid (iii) [500 MHz, CDCl3], Figure S19. Mass spectrum of β-sitosterol (4), Effigy S20. Mass spectrum of stigmasterol (v), Figure S21. 1H-NMR spectrum of the mixture of β-sitosterol (4) and stigmasterol (5) [700 MHz, CDCl3], Figure S22. 13C-NMR spectrum of the mixture of β-sitosterol (4) and stigmasterol (5) [175 MHz, CDClthree], Figure S23. 1H→13C HSQC NMR spectrum of β-sitosterol and stigmasterol [700 MHz, CDCl3], Effigy S24. iH→13C HMBC NMR spectrum of β-sitosterol (4) and stigmasterol (v) [700 MHz, CDCl3], Figure S25. 1H→1H COSY NMR spectrum of β-sitosterol (4) and stigmasterol (5) [700 MHz, CDCl3], Figure S26. Mass spectrum of one-octadecanol (6), Figure S27. 1H-NMR of 1-octadecanol (6) [700 MHz, CDCl3], Effigy S28. 13C-NMR spectrum of 1-octadecanol (6) [175 MHz, CDClthree], Effigy S29. 1H→thirteenC HSQC NMR spectrum of 1-octadecanol (six) [700 MHz, CDCl3], Figure S30. 1H→thirteenC HMBC NMR spectrum of 1-octadecanol (half dozen) [700 MHz, CDCl3 ], Figure S31. 1H→1H COSY NMR spectrum of 1-octadecanol (6) [700 MHz, CDCl3].
Author Contributions
H.A.W. designed the research projection and supervised the progress. N.A.Northward.A., and Thou.B.A., contributed equally to the manuscript and wrote the main manuscript text. Northward.A.N.A., M.B.A., and M.S.A.R. performed the isolation, structure elucidation, in vitro and in silico molecular docking and analyzed all information. R.D., T.North. and A.Chiliad.G. supervised the study. All authors read and canonical the last manuscript.
Funding
This study was supported financially by USM RU Height-DOWN projection entitled Catalogue of USM-RIKEN Natural Product (CURINaP) Library for the Discovery of Bioactive Molecules on Ageing and Ageing- Related Diseases, 1001/PFARMASI/870031.
Conflicts of Interest
The authors declare no disharmonize of involvement.
References
- Jalwal, P.; Middha, A.; Ramchander, C. Recent advances on senna equally a laxative: A comprehensive review. J. Pharm. Phytochem. 2017, 6, 349–353. [Google Scholar]
- Lavanya, B.; Maheswaran, A.; Vimal, N.; Vignesh, Grand.; Uvarani, K.Y.; Varsha, R. An overall view of cassia species phytochemical constituents and its pharmacological uses. Int. J. Pharm. Sci. Res. 2018, 3, 47–fifty. [Google Scholar]
- Kaur, I.; Ahmad, South.; Harikumar, S.50. Pharmacognosy, phytochemistry and pharmacology of Cassia occidentalis Linn. Int. J. Pharm. Phytochem. Res. 2014, six, 151–155. [Google Scholar]
- Feitosa, C.1000.; Freitas, R.Chiliad.; Luz, Due north.N.N.; Bezerra, One thousand.Z.B.; Trevisan, M.T.S. Acetylcholinesterase inhibition by somes promising Brazilian medicinal plants. Braz. J. Boil. 2011, 71, 783–789. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Shukla, V.J. Antibacterial and antifungal action from flower extracts of Cassia fistula 50.: An ethnomedicinal plant. Int. J. PharmTech Res. 2011, 3, 160–168. [Google Scholar]
- Sundaramoorthy, S.; Gunasekaran, S.; Arunachalam, S.; Sathiavelu, M. A Phytopharmacological Review on Cassia Species. J. Pharm. Sci. Res. 2016, eight, 260–264. [Google Scholar]
- Bhalodia, N.; Acharya, R.; Shukla, Five. Evaluation of in vitro Antioxidant Activity of hydro-alcoholic seed extratcs of Cassia fistula linn. Gratis Radic. Antioxid. 2011, 1, 68–76. [Google Scholar] [CrossRef]
- Balkrishna, A.; Misra, L.N. Ayurvedic Plants in Encephalon Disorders: The Herbal Hope. J. Tradit. Med. Clin. Nat. 2017, six, 1–9. [Google Scholar] [CrossRef]
- Deshpande, H.A.; Bhalsing, Southward.R. Contempo Advances in the phytochemistry of some medicinally important Cassia species: A review. Int. J. Pharma Med. Biol. Sci. 2013, 2, 60–78. [Google Scholar]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer'due south Affliction. Trends Pharm. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Borloz, A.; Urbain, A.; Marston, A.; Hostettmann, K.; Carrupt, P.-A.; Reist, M. In vitro screening assays to place natural or synthetic acetylcholinesterase inhibitors: Thin layer chromatography versus microplate methods. Eur. J. Pharm. Sci. 2008, 33, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, S.; Zahid, One thousand.; Parveen, South.; Ahmad, Z.; Singh, P.Thou.; Parveen, Z. Alzheimer'south illness: Delivery of drugs through intrasanal route. J. Drug Deliv. Ther. 2016, 6, 60–69. [Google Scholar] [CrossRef]
- Moyo, Yard.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. 2010, 123, 69–76. [Google Scholar] [CrossRef]
- Craig, 50.A.; Hong, N.South.; McDonald, R.J. Neuroscience and Biobehavioral Reviews Revisiting the cholinergic hypothesis in the development of Alzheimer'southward illness. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C., Jr.; Bolzani, 5.S.; Pimentel, L.S.; Castro, N.Thou.; Cabral, R.F.; Costa, R.S.; Floyd, C.; Rocha, M.Southward.; Young, K.C.K.; Barreiro, E.J.; et al. New selective acetylcholinesterase inhibitors designed from natural piperidine alkaloids. Bioorgan. Med. Chem. 2005, 13, 4184–4190. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S.; Kamal, M.A. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and futurity prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Danish, M.; Singh, P.; Mishra, G.; Srivastava, S.; Jha, 1000.K.; Khosa, R.50. Cassia fistula Linn (Amulthus)—An Of import Medicinal Institute: A Review of Its Traditional Uses, Phytochemistry and Pharmacological Properties. J. Nat. Prod. Constitute Resour. 2011, i, 101–118. [Google Scholar]
- Dave, H.; Ledwani, 50. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Singh, S.; Singh, S.Grand.; Yadav, A. A Review on Cassia species: Pharmacological, Traditional and Medicinal Aspects in Various Countries. Am. J. Phytomed. Clin. 2013, 1, 291–312. [Google Scholar]
- Senna siamea (Lam.) H.S. Irwin & Barneby. The Institute List—A Working Listing of All Plant Species. Bachelor online: http://www.theplantlist.org/tpl/tape/ild-1117 (accessed on 14 February 2019).
- Gritnasapan, W.; Tantisewie, B.; Vichiara, J. Chemic Constituents of Cassia timorensis and Cassia grandis. J. Sci. Soc. Thail. 1984, ten, 189–190. [Google Scholar] [CrossRef]
- Thongsaard, Due west.; Pongsakorn, South.; Sudsuang, R.; Bennett, Grand.; Kendall, D.A.; Marsden, A.; Barakol, C. A natural anxiolytic, inhibits striatal dopamine release but off uptake in vitro. Eur. J. Pharm. 1997, 319, 157–164. [Google Scholar] [CrossRef]
- Sukma, M.; Chaichantipyuth, C.; Murakami, Y.; Tohda, M.; Matsumoto, K.; Watanabe, H. CNS inhibitory effects of barakol, a constituent of Cassia siamia Lamk. J. Ethnopharmacol. 2002, 83, 87–94. [Google Scholar] [CrossRef]
- Li, G.; Shao, 1000.; Umeshappa, C.Due south. Recent progress in blood-brain barrier transportation research. In Encephalon Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Bookish Press: London, UK, 2019; pp. 33–51. [Google Scholar]
- Thakur, R.Due south.; Ahirwar, B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J. Food Drug Anal. 2018, 27, 231–239. [Google Scholar] [CrossRef]
- Irshad, K.; Mehdi, S.J.; Al-Fatlawi, A.A.; Zafaryab, M.; Ali, A.; Ahmad, I.; Singh, Thousand.; Rizvi, M.K.A. Phytochemical Composition of Cassia fistula Fruit Extracts and its Anticancer Activity Against Human being Cancer Cell Lines. J. Boil. Act. Prod. Nat. 2014, 4, 158–170. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Ali, A.Thou.; Aboul-Enein, S.Chiliad.; Mohamed, F.M.; Abou, E.; Magdy, Thou.D.; Mohammed, A.R.H. Phytochemical, cytotoxicity and antioxidant investigation of Cassia alata leaves growing in Egypt. J. Innov. Pharm. Biol. Sci. 2017, iv, 97–105. [Google Scholar]
- Chaturvedula, Five.S.P.; Prakash, I. Isolation of Stigmasterol an ?-Sitosterol from the dichloromethane excerpt of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Rowshanul, H.M.; Farjana, N.; Matiar, R.; Ekramul, H.M.; Rezaul, Grand.M. Isolation of stigmasterol and β-sitosterol from methanolic extract of root. Pak. J. Biol. Sci 2007, ten, 4174–4176. [Google Scholar]
- Kamboj, A.; Saluja, A.K. Isolation of stigmasterol and β-sitosterol from petroleum ether extract of aeriform parts of Ageratum conyzoides (Aster family). Int. J. Pharm. Pharm. Sci. 2011, 3, 94–96. [Google Scholar]
- Malhotra, S.; Misra, K. Anthraquinones from Cassia sophera heartwood. Phytochemistry 1982, 21, 197–199. [Google Scholar] [CrossRef]
- Beulah, K.G.; Soris, P.T.; Mohan, 5.R. GC-MS Decision of Bioactive Compounds of Dendrophthoe falcata (L.F) Ettingsh: An Epiphytic Plant. Int. J. Wellness Sci. Res. 2018, 8, 261–269. [Google Scholar]
- Verma, R.N.; Batra, A. Isolation and analytic label of rebaudioside A and GC-MS assay of methanolic leaves extract of Stevia rebaudiana Bert. Ann. Phytomed. 2013, 2, 108–114. [Google Scholar]
- Geetha, D.; Rajeswari, Grand.; Jayashree, I.; Qu, S. Chemical profiling of Elaeocarpus serratus L. by GC-MS. Asian Pac. J. Trop. Biomed. 2013, 3, 985–987. [Google Scholar] [CrossRef]
- Khan, I.; Zahoor, K.; Zeb, A.; Sahibzada, M.U.K.; Bari, Westward.U.; Naz, S. Isolation, characterization, pharmacological evaluation and in silico modeling of bioactive secondary metabolites from Ziziphus oxyphylla a member of Rhamnaceae family. Trop. J. Pharm. Res. 2020, 19, 351–359. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid send across RBE4 cells: A blood-brain barrier model. Cell. Mol. Boil. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Végh, Yard.; Riethmüller, Due east.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, A.; Toth, A.; Béni, Southward.; Könczöl, Á.; Balogh, G.T.; et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Bulwark. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, Due north.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, xviii, 1685. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, Thousand.I. Flavonoids equally acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef]
- Balkis, A.; Tran, M.; Lee, Y.Z.; Ng, Grand. Screening Flavonoids for Inhibition of Acetylcholinesterase Identified Baicalein as the Near Potent Inhibitor. J. Agric. Sci. 2015, vii, 26–35. [Google Scholar] [CrossRef]
- Čolović, M.; Krstić, D.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Choi, S.; Tan, S.; Wahab, H.A.; Chan, One thousand.; Murugaiyah, V. Prenylated xanthones from mangosteen every bit promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 2014, 21, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Dvir, H.; Silman, I.; Harel, Chiliad.; Rosenberry, T.L.; Sussman, J.50. Acetylcholinesterase: From 3D structure to function. Chem.-Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Neto, D.C.F.; de Souza Ferreira, Grand.; da Conceição Petronilho, Due east.; Lima, J.A.; de Azeredo, S.O.F.; de Oliveira Carneiro Brum, J.; exercise Nascimento, C.J.; Villar, J.D.F. A new guanylhydrazone derivative as a potential acetylcholinesterase inhibitor for Alzheimer's disease: Synthesis, molecular docking, biological evaluation and kinetic studies by nuclear magnetic resonance. RSC Adv. 2017, 7, 33944–33952. [Google Scholar] [CrossRef]
- Greenblatt, H.One thousand.; Guillou, C.; Guénard, D.; Argaman, A.; Botti, Southward.; Badet, B.; Thal, C.; Silman, A.I.; Sussman, J.L. The Circuitous of a Bivalent Derivative of Galanthamine with Torpedo Acetylcholinesterase Displays Desperate Deformation of the Active-Site Gorge: Implications for Structure-Based Drug Design. J. Am. Chem. Soc. 2004, 126, 15405–15411. [Google Scholar] [CrossRef]
- Morris, G.Thousand.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.Due south.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, thirty, 2785–2791. [Google Scholar] [CrossRef]
| Sample Availability: Samples of the compounds are not available from the authors. |
Figure ane. The structures of the isolated compounds; ane–6 of C. timorensis leaves and flowers.
Figure 1. The structures of the isolated compounds; ane–vi of C. timorensis leaves and flowers.
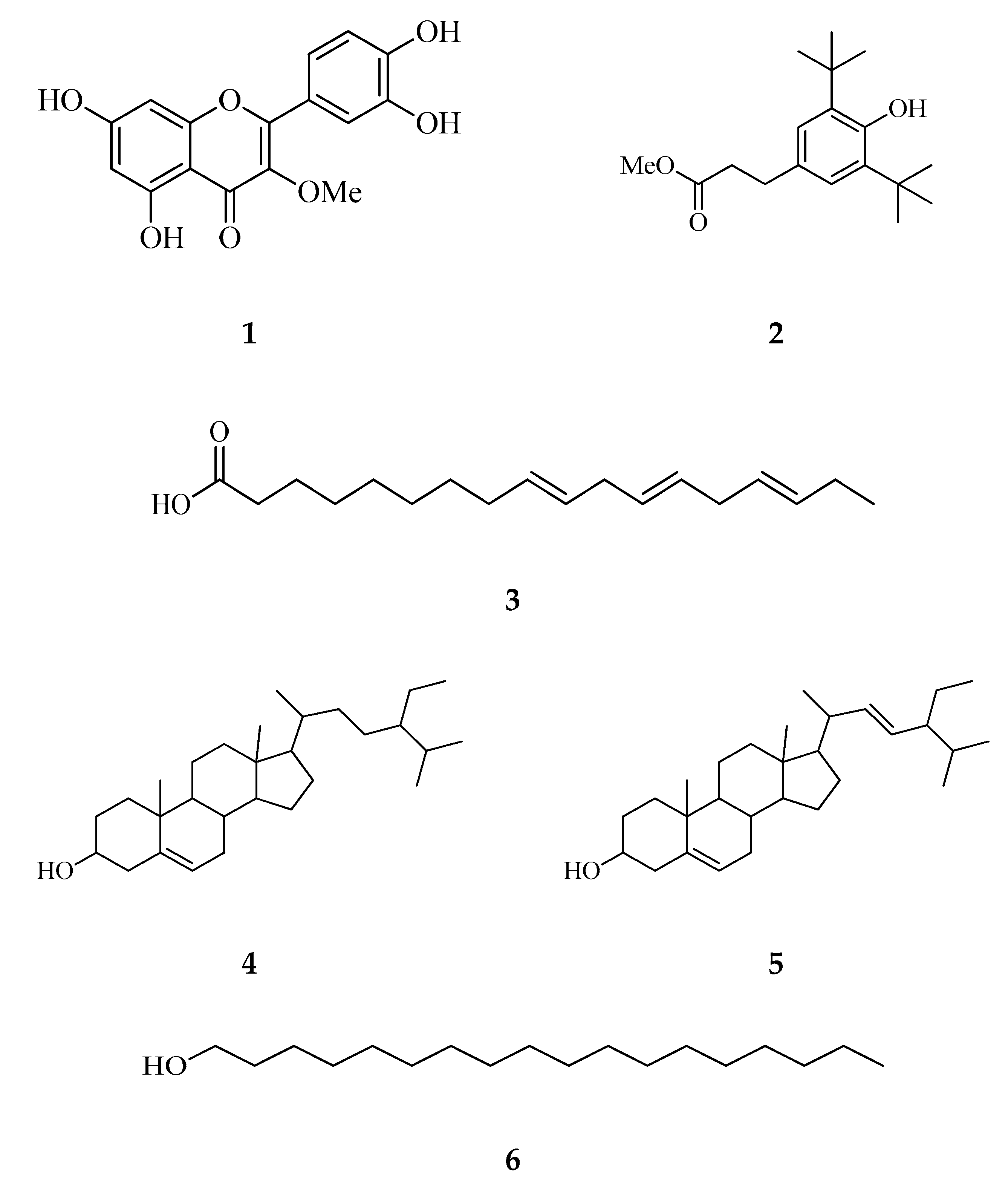
Effigy two. HMBC and COSY primal correlations of compounds 1–6.
Effigy 2. HMBC and COSY key correlations of compounds one–6.
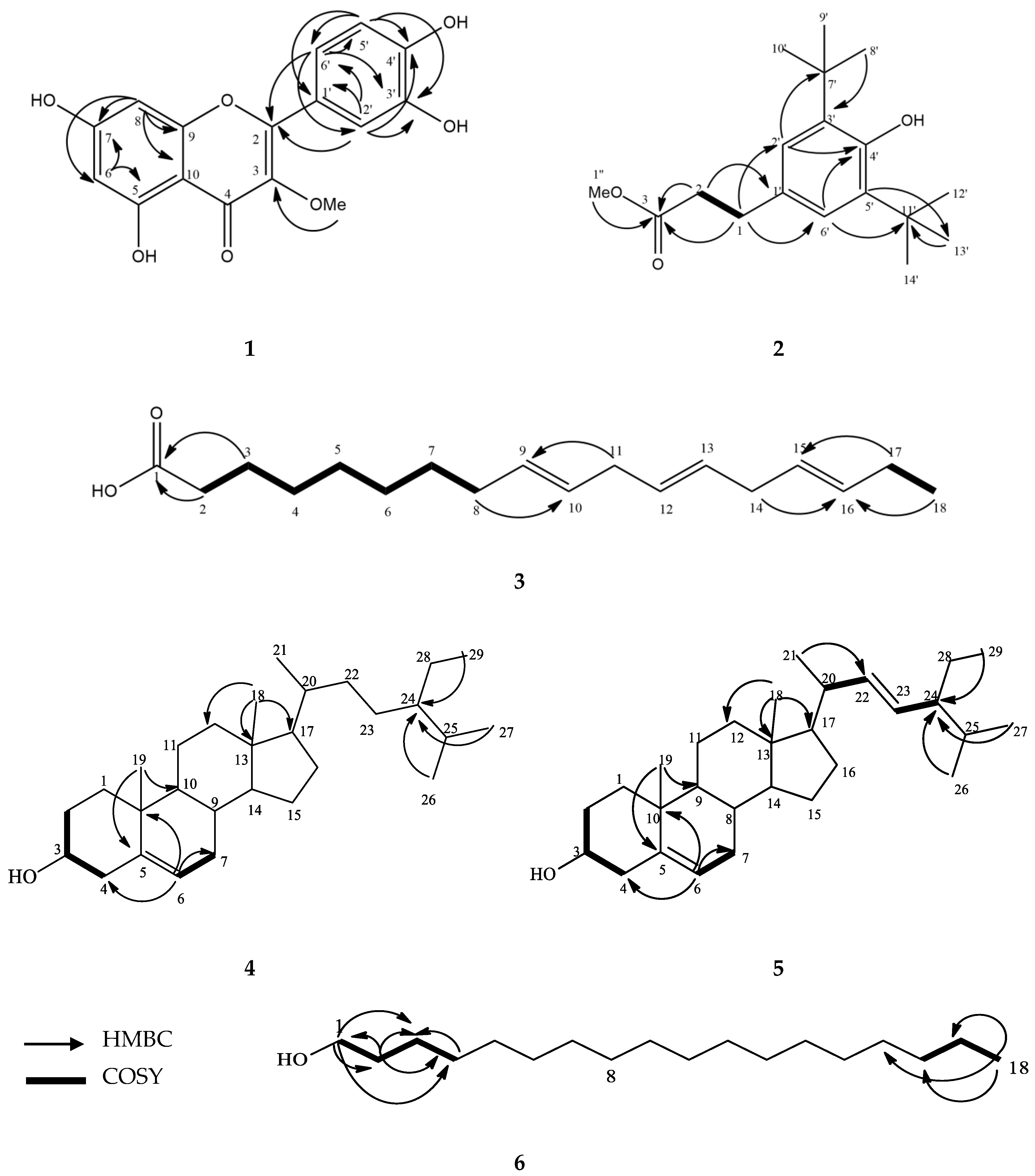
Figure iii. Interactions of compound (a) galanthamine, (b) iii-methoxy quercetin, (c) β-sitosterol, and (d) stigmasterol with PAS (amino acid in green) and the acyl pocket (amino acrid in carmine) in the active site of TcAChE. The dotted green line is the hydrogen bond, purple is the Pi-sigma bail, and pink is the Pi-alkyl bail interaction.
Figure 3. Interactions of compound (a) galanthamine, (b) iii-methoxy quercetin, (c) β-sitosterol, and (d) stigmasterol with PAS (amino acrid in green) and the acyl pocket (amino acid in reddish) in the active site of TcAChE. The dotted green line is the hydrogen bond, purple is the Pi-sigma bond, and pink is the Pi-alkyl bond interaction.
Tabular array 1. Percentage inhibition of methanol extracts of Cassia spp. at 0.two mg/mL.
Table 1. Percentage inhibition of methanol extracts of Cassia spp. at 0.ii mg/mL.
| Plants | Part | Per centum Inhibition (%) one | Strength of Inhibition |
|---|---|---|---|
| C. timorensis | Leaves | 94.69 ± three.08 | Skilful |
| C. timorensis | Stems | 97.16 ± two.08 | Good |
| C. timorensis | Flowers | 96.94 ± 0.73 | Good |
| C. timorensis | Fruits | 49.09 ± i.64 | Moderate |
| C. grandis | Leaves | 86.09 ± 0.66 | Good |
| C. grandis | Stems | 91.76 ± 0.73 | Practiced |
| C. fistula | Leaves | 20.63 ± 6.68 | Poor |
| C. fistula | Stems | 64.32 ± 0.09 | Good |
| C. fistula | Flowers | 8.72 ± 1.x | Poor |
| C. fistula | Fruits | nineteen.48 ± 2.86 | Poor |
| C. spectabilis | Leaves | 45.96 ± viii.20 | Moderate |
| C. spectabilis | Stems | 27.18 ± four.79 | Moderate |
| C. spectabilis | Fruits | 37.32 ± 2.07 | Moderate |
| C. alata | Leaves | 26.40 ± 6.65 | Moderate |
| C. alata | Stems | 18.43 ± vii.42 | Poor |
| C. alata | Flowers | 29.01 ± three.05 | Moderate |
| C. alata | Fruits | 27.86 ± one.63 | Moderate |
Tabular array 2. Percentage inhibitions of C. timorensis fractions at 0.2 mg/mL.
Table 2. Percentage inhibitions of C. timorensis fractions at 0.ii mg/mL.
| Plants | Fractions | Percent Inhibition (%) 1 |
|---|---|---|
| C. timorensis leaves | Hexane | 30.92 ± 4.92 |
| Ethyl acetate | 98.98 ± 0.57 | |
| Butanol | 91.83 ± 1.fifty | |
| Aqueous | 98.80 ± 0.55 | |
| C. timorensis stems | Hexane | 26.66 ± one.70 |
| Ethyl acetate | 30.84 ± 2.83 | |
| Butanol | 94.92 ± 1.sixty | |
| Aqueous | 84.35 ± 1.26 | |
| C. timorensis flowers | Hexane | 92.35 ± 0.01 |
| Ethyl acetate | 95.98 ± 1.thirty | |
| Butanol | 98.xvi ± 0.05 | |
| Aqueous | 38.32 ± 0.09 |
Tabular array iii. ICfifty values of compound 1 against Ache compared to quercetin and galantamine.
Table 3. ICfifty values of chemical compound 1 against Ache compared to quercetin and galantamine.
| Compound | AChE Inhibition (IC50) µM |
|---|---|
| 1 | 83.71 ± 4.67 |
| Quercetin | 249.10 ± 27.14 |
| Galantamine | 4.63 ± 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open up access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/four.0/).
Source: https://www.mdpi.com/1420-3049/25/19/4545/htm
 ,
,
0 Response to "A Review on Anthraquinones Isolated From Cassia Spcies Adn Their Applications"
Post a Comment